Density of Common Metals and Alloys

This article presents tables to detail the density, property and application of common metals and alloys for your references.
What is Density?
Density is a fundamental property of matter that measures how much mass is packed into a given volume of a substance.
It indicates how tightly atoms or molecules are arranged in a material. The formula to calculate density is:
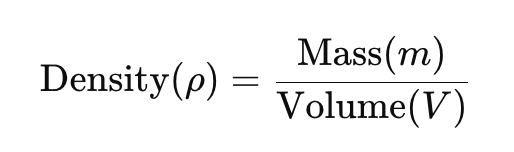
In simpler terms, density is the amount of mass per unit volume. Denser materials have more mass packed into a smaller space, while less dense materials are more spread out.
Unit of Density
The most common unit for measuring density is grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³), depending on the system being used:
In the metric system, density is often expressed in g/cm³ or kg/m³.
In the Imperial system, it may be expressed as pounds per cubic foot (lb/ft³). For most metals, density is typically represented in g/cm³ because it's a convenient unit for solid materials.
Factors Influencing Density
Several factors can influence the density of a material:
Atomic Mass: the heavier atoms, the higher density.
Materials made of heavier atoms tend to have higher densities. For example, gold (Au) atoms are much heavier than aluminum (Al) atoms, so gold is denser.
Atomic Packing Structure: the tighter, the denser.
The way atoms are arranged in a material also affects its density. Materials with tightly packed atomic structures (such as metals with face-centered cubic (FCC) structures) are generally denser.
Temperature: the higher temperature, the higher density.
As temperature increases, most materials expand, increasing their volume and decreasing their density. Conversely, cooling a material usually increases its density.
Pressure: minimal change in density unless under very high pressure
In general, increasing pressure increases density, while decreasing pressure decreases density. This relationship is more pronounced in gases but also applies to solids and liquids under certain conditions.
Incompressibility: Liquids and solids are much less compressible than gases. This means that under normal pressures, their density changes very little when pressure is applied.
Extreme Pressure: At extremely high pressures, even solids and liquids can experience a slight increase in density, as the atoms or molecules are forced closer together.
To help you better understand the influence of pressure, we have provided an example of the density change of solid aluminum under different pressure values.
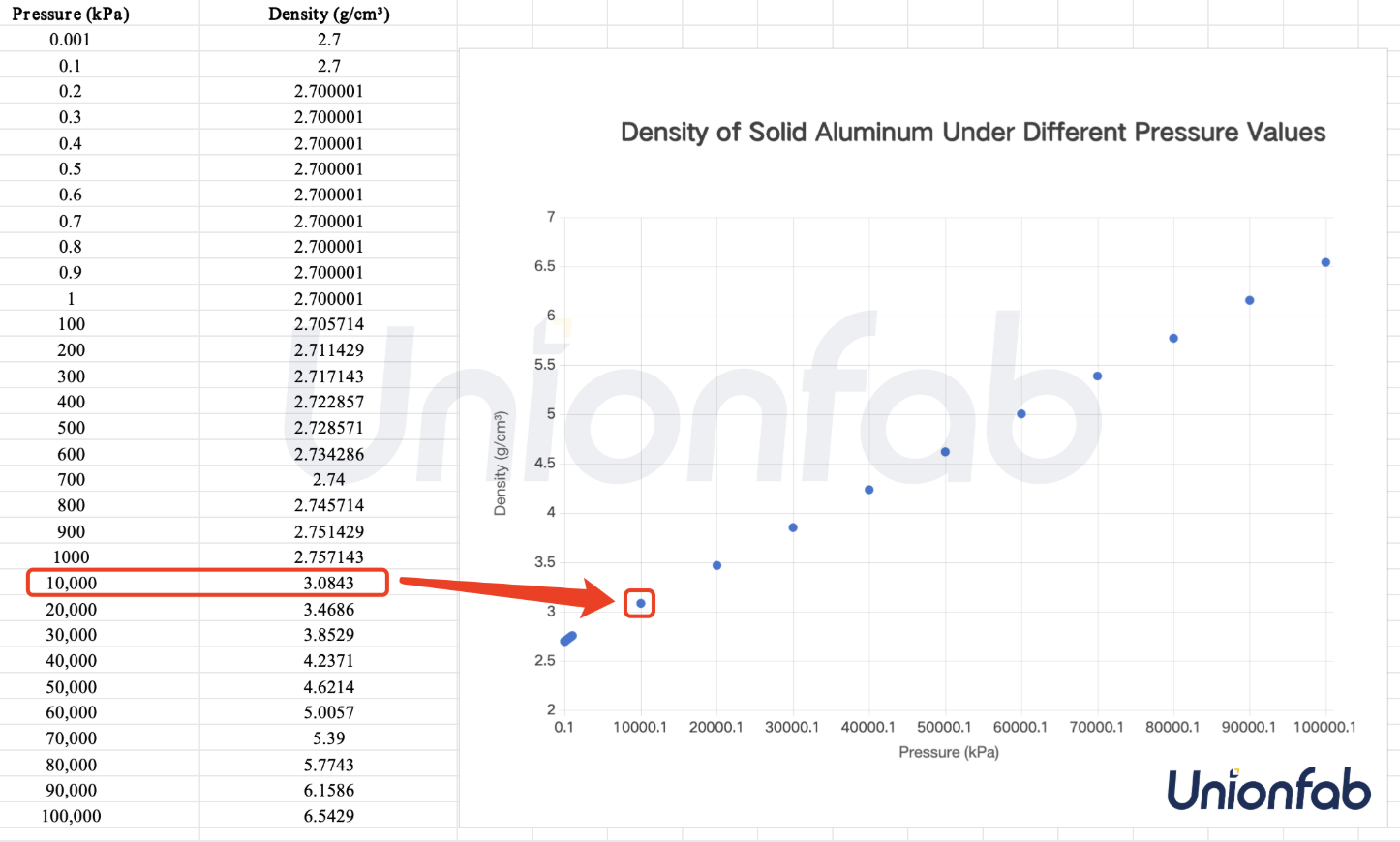
As we can see from the graph here, the density of aluminum rarely increases when the pressure is under 10000 Kpa, or 10Gpa, and undergoes significant volume changes in the range of several GPa to tens of GPa.
Density of Common Metals
Here’s a breakdown of the densities of some common metals.
Metal | Density (Ascending) | Description |
|---|---|---|
| 2.7 g/cm³; | Known for its low density and used in aerospace and automotive industries for lightweight materials. |
| 4.5 g/cm³; | Valued for its high strength-to-weight ratio, corrosion resistance, and used in aerospace and medical applications. |
| 7.19 g/cm³; | Known for its hardness and corrosion resistance, often used as a protective coating for metals. |
| 7.87 g/cm³; | Commonly used in construction and manufacturing; steel, an alloy of iron, is widely utilized. |
| 8.9 g/cm³; | Used in high-strength alloys and magnets, valued for its wear resistance and magnetic properties. |
| 8.9 g/cm³; | Valued for corrosion resistance, often used in stainless steel and other alloys. |
| 8.96 g/cm³; | Known for its excellent electrical and thermal conductivity, widely used in electrical wiring and plumbing. |
| 10.5 g/cm³; | Valued for its electrical conductivity and reflective properties, significantly denser than aluminum. |
| 19.25 g/cm³; | Extremely dense, known for its high melting point and used in applications requiring high heat resistance. |
| 19.3 g/cm³; | One of the densest metals, prized for jewelry and electronics due to malleability and corrosion resistance. |
In summary, among the above 10 mentioned metals, Aluminum is the lightest and Gold is the heaviest under the same conditions.
Density of Common Alloys
Alloys are mixtures of two or more metals, and their density depends on the density and amount of each metal used.
Usually, if a base metal is mixed with a denser metal, the alloy will likely be heavier, or has higher density, and if it's mixed with a lighter metal, the alloy will be lighter, or has lower density.
In terms of the added amount, the more of the low-density material you add, the more it "pulls down" the overall density of the alloy. Similarly, if a higher-density metal is added in larger proportions, it will increase the overall density.
Here we have draw a chart to help you understand better.
*Note: The values and the rate of increase in this chart hold no reference value and are solely intended to assist in illustrating the growth trend of the curve.
The following table presents the details of some common alloys.
Alloy Type | Base Metal | Common Types | Composition | Density (g/cm³) | Change in Density | Properties | Applications |
|---|---|---|---|---|---|---|---|
Steel | Fe - 7.87 g/cm³ | Carbon Steel | Fe, up-to-2.1% C (2.267 g/cm³) | 7.85 | Slightly lower than pure Fe | Strong, ductile, affordable | Construction, automotive, tools |
Alloy Steel | Fe, Cr (7.19 g/cm³), V (6.11 g/cm³), Ni (8.9 g/cm³) | 7.85 | Slightly lower than pure Fe | High strength, wear-resistant | Machinery, pipelines, automotive parts | ||
Stainless Steel | Fe - 7.87 g/cm³ | 304 Stainless Steel | Fe, 18-20% Cr (7.19 g/cm³), 8-10.5% Ni (8.9 g/cm³) | 7.75–8.1 | Slightly lower or higher than pure Fe | Corrosion-resistant, durable | Kitchen equipment, medical devices, chemical tanks |
316 Stainless Steel | Fe, 16-18% Cr (7.19 g/cm³), 10-14% Ni (8.9 g/cm³), 2-3% Mo (10.28 g/cm³) | 7.75–8.1 | Higher density than 304 due to added Mo | Superior corrosion resistance, high strength | Marine equipment, chemical processing, medical implants | ||
Brass | Cu - 8.96 g/cm³ | Cartridge Brass | 70% Cu, 30% Zn (7.14 g/cm³) | 8.4–8.7 | Lower than pure Cu | Malleable, corrosion-resistant | Ammunition, instruments, plumbing |
Admiralty Brass | 70% Cu, 29% Zn (7.14 g/cm³), 1% Sn (7.31 g/cm³) | 8.4–8.7 | Slightly higher density due to Sn | Excellent corrosion resistance, easy to machine | Marine hardware, condensers, pipes | ||
Bronze | Cu - 8.96 g/cm³ | Phosphor Bronze | 88-90% Cu (8.96 g/cm³), 8-10% Sn (7.31 g/cm³), 0.2% P (1.82 g/cm³) | 8.7–8.9 | Higher than brass, lower than Cu | Strong, fatigue-resistant, low friction | Springs, bearings, electrical connectors |
Aluminum Bronze | 88-92% Cu (8.96 g/cm³), 6-12% Al (2.70 g/cm³) | 8.7–8.9 | Slightly lower density than phosphor bronze | High strength, corrosion-resistant | Marine hardware, heavy machinery parts | ||
Aluminum Alloys | Al - 2.70 g/cm³ | 6061 Aluminum | Al 87.8-88.8% (2.70 g/cm³), Mg 0.8-1.2% (1.74 g/cm³), Si 0.4-0.8% (2.33 g/cm³) | 2.6–2.9 | Slightly higher than pure Al | Lightweight, weldable, good mechanical properties | Aerospace, automotive, structural components |
7075 Aluminum | 87.4-88.8% Al (2.70 g/cm³), 5.1-6.1% Zn (7.14 g/cm³), 2.1-2.9% Mg (1.74 g/cm³) | 2.6–2.9 | Higher density due to Zn | High strength, good fatigue resistance | Aerospace, military, high-stress components | ||
Titanium Alloys | Ti - 4.50 g/cm³ | Ti-6Al-4V | 90% Ti (4.50 g/cm³), 6% Al (2.70 g/cm³), 4% V (6.11 g/cm³) | 4.43–4.5 | Slightly higher than pure Ti | Lightweight, high strength, corrosion-resistant | Aerospace, medical implants, sports equipment |
Nickel Alloys | Ni - 8.90 g/cm³ | Inconel 625 | 58% Ni (8.90 g/cm³), 20-23% Cr (7.19 g/cm³), 8-10% Mo (10.28 g/cm³) | 8.2–8.9 | Higher than pure Ni | High temperature resistance, corrosion-resistant | Aerospace, chemical processing, extreme environments |
Monel 400 | 63% Ni (8.90 g/cm³), 28-34% Cu (8.96 g/cm³) | 8.2–8.9 | Slightly lower than Inconel | Strong, corrosion-resistant, good mechanical properties | Marine equipment, valves, chemical processing |
To find the density value of more metals and alloys, please refer to the article: Density of metal and alloys.
Metals and Alloys Used in 3D Printing
Except for the applications like automotive, aerospace, and tooling etc. mentioned above, metals and alloys are also widely applied in 3D Printing.
Metal 3D printing, has become increasingly popular in industries such as aerospace, medical, and automotive due to its ability to produce strong, lightweight, and geometrically intricate parts. Here we have listed the commonly-used metals and alloys in 3d printing for your reference.
Alloy | Grade | Properties | Applications |
|---|---|---|---|
Stainless Steel | Corrosion-resistant, durable, strong, high-temperature resistance | Medical implants, chemical tanks, marine applications | |
Precipitation-hardened, high strength, corrosion-resistant | Aerospace parts, food processing, industrial equipment | ||
Aluminum Alloys | Lightweight, corrosion-resistant, good thermal properties | Aerospace components, automotive parts, heat exchangers | |
High strength, weldable, good mechanical properties | Structural components, automotive frames, marine structures | ||
Titanium Alloys | Ti-6Al-4V | Lightweight, strong, corrosion-resistant, biocompatible | Medical implants, aerospace parts, sports equipment |
Nickel Alloys | Inconel 625 | High-temperature resistance, corrosion-resistant, strong | Jet engines, turbines, extreme environment components |
Inconel 718 | High strength, temperature stability, excellent mechanical properties | Aerospace, power generation, oil & gas | |
Cobalt-Chrome Alloys | CoCrMo | Wear-resistant, biocompatible, corrosion-resistant | Medical implants, dental prosthetics, aerospace parts |
Copper Alloys | Pure Copper | Excellent electrical conductivity, thermal conductivity | Electrical wiring, heat exchangers, plumbing systems |
CuCrZr | High strength, conductivity, and resistance to softening | Heat sinks, electrical components, welding electrodes | |
Tool Steels | H13 | High hardness, wear-resistant, heat-tolerant | Molds, dies, cutting tools, high-temperature applications |
A2 | Air-hardening, tough, wear-resistant | Punches, dies, industrial tools | |
D2 | High wear resistance, high hardness, good dimensional stability | Blades, cutting tools, industrial tooling |
If you're looking for top-notch metal 3D printing, Unionfab has you covered! Here are four key reasons to choose Unionfab for all your manufacturing needs:
Expertise: Over 20 years of experience in 3D printing, backed by a fleet of 100 3D printers.
Quality Assurance: Certified to the strict ISO 9001 quality management standards.
Fast Shipping: Get your parts delivered in just 3-5 days with reliable air shipping via DHL Express and FedEx.
Competitive Pricing: Up to 70% cheaper than European and American competitors without compromising on quality. Feel free to CONTACT US for any of your 3D printing projects!

FAQs
Which metal has the highest density?
Osmium (22.59 g/cm³) is a bluish-white metal and the densest naturally occurring element. It’s extremely hard and has a high melting point.
Due to its density and corrosion resistance, it’s used in applications like fountain pen tips, electrical contacts, and alloys for extreme wear resistance.
Which metal is the lowest density?
Under standard conditions, Lithium (0.534 g/cm³) is the lightest metal and has important applications in rechargeable batteries (such as in smartphones and electric vehicles).
It’s highly reactive, so it's usually stored in mineral oil. Lithium also has a high electrochemical potential, making it ideal for energy storage solutions.
What are the top 5 lightest metals?
|
| *Strength-to-weight Ratio (MPa/(g/cm³)) |
|
|
|
|
|---|---|---|---|---|---|---|
Lithium | 0.534 | ≈42 | Low | Medium | Lightest metal, high electrochemical potential | Rechargeable batteries, energy storage solutions |
Potassium | 0.862 | ≈8 | Low | Low | Soft, reacts easily with water | Fertilizers, chemical production |
Sodium | 0.971 | ≈9 | Low | Low | Highly reactive, commonly found in salts | Chemical industries, salt production |
Magnesium | 1.738 | ≈158 | Medium | Medium | Lightweight yet strong, corrosion-resistant | Automotive and aerospace components, electronics, medical implants |
Calcium | 1.55 | ≈20 | Low | Low | Used as an alloying agent in industrial processes | Aluminum and steel production, alloys for improving strength |
*Strength-to-weight Ratio: This ratio shows how strong a metal is compared to its weight. A higher number means the metal is stronger without being too heavy, making it good for things that need to be strong but light.
What metal is strong but light?
Titanium (4.5 g/cm³) is known for its exceptional strength-to-weight ratio.
It’s much lighter than steel but just as strong, making it a popular choice in industries like aerospace, medical devices (like implants), and sports equipment. Titanium is also highly resistant to corrosion, even in extreme environments like seawater.

